The XU group aims to develop transformations that are genuinely practical and useful in the realm of organic synthesis. Our goal is to exploit new strategies and invent novel methodologies that can simplify access privileged chemical architectures, enabling the direct construction of complex molecules from readily available and abundant feedstocks. Additionally, we are committed to thoroughly interrogate the mechanisms to gain a deep understanding of the reactions.
The current research in the XU's group focuses on these two parts:
Part I: Deoxygenative Difunctionalization of Carbonyls (DODC)
Carbonyl compounds (aldehydes and ketones) have many advantages in terms of availability, universality, environmental friendliness, and late-stage modification. Although a number of transformations based on carbonyls have been well established, as illustrated by many named reactions, such as Grignard reaction, Wittig reaction, Wolff-Kishner-Huang reaction, and so on, the much more challenging deoxygenative functionalization, especially deoxygenative difunctionalization, is still very less studied. DODC provides a novel route to directly introduce two functional groups into carbonyl sites. Thus, this research is highly rewarding due to the straightforward and convenient conversion of the carbonyl compounds into dual-functionalized skeletons, which can be further transferred into complex molecules within just a few steps.

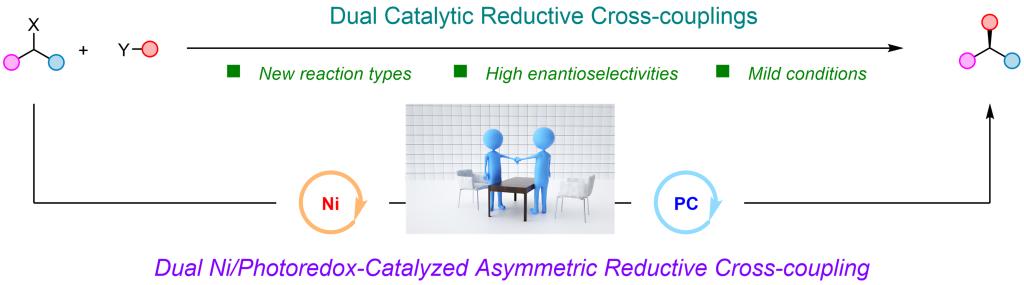
Part II: Dual Catalytic Reductive Cross-coupling
In this field, we mainly put our attentions on dual nickel/photoredox-catalyzed asymmetric reductive cross-coupling reactions. Asymmetric cross-couplings, which can be incorporated with high efficiency into the protocol to generate chiral carbon centers, have emerged as a vibrant field of research. Compared with traditional asymmetric cross-couplings or reductive cross-couplings with a single nickel catalyst, we aim to take the efforts on the unsolved transformations by using the dual nickel/photoredox catalysis regime. We have demonstrated that this dual catalytic system can be successfully implemented in several couplings that were unfeasible or difficult to achieve with previous patterns. All these results underscore the unique advantages of this approach. Recently, our efforts have focused on achieving asymmetric reactions using alcohols as coupling reagents within this dual catalysis system, by employing the strategies we have developed for C-O bond transformation.